ACCESS Award
Award to Catalyze Care through Equitable, Scalable Solutions
Background
Cancer remains a leading cause of death worldwide, with stark disparities in access to effective treatment. Innovative, affordable, and scalable solutions are urgently needed to improve outcomes and equity, particularly in low- and middle-income countries (LMICs).
Scope and Objectives
We invite proposals that address the central question:
How can low-cost, scalable interventions—such as AI-driven tools, drug repurposing, and dose optimization—transform cancer care by improving treatment options and expanding access for patients worldwide?
Trials should:
- Demonstrate clinical relevance and potential for rapid implementation.
- Prioritize cost-effectiveness and scalability in diverse health systems.
- Include strategies for patient-centered outcomes.
Eligibility
- Academic institutions, non-profit organizations, and consortia.
- Strong preference for international collaborations and inclusion of LMIC centers.
- Proposals must include patient partners– they play an active governance role in the project, meaning they are responsible for specific project tasks and maintain direct communication with Rising Tide about reporting project activities, similar to the Principal Investigator (PI). Throughout the project, they take ownership of their development as co-PIs, exercising agency and leadership.
Funding and Duration
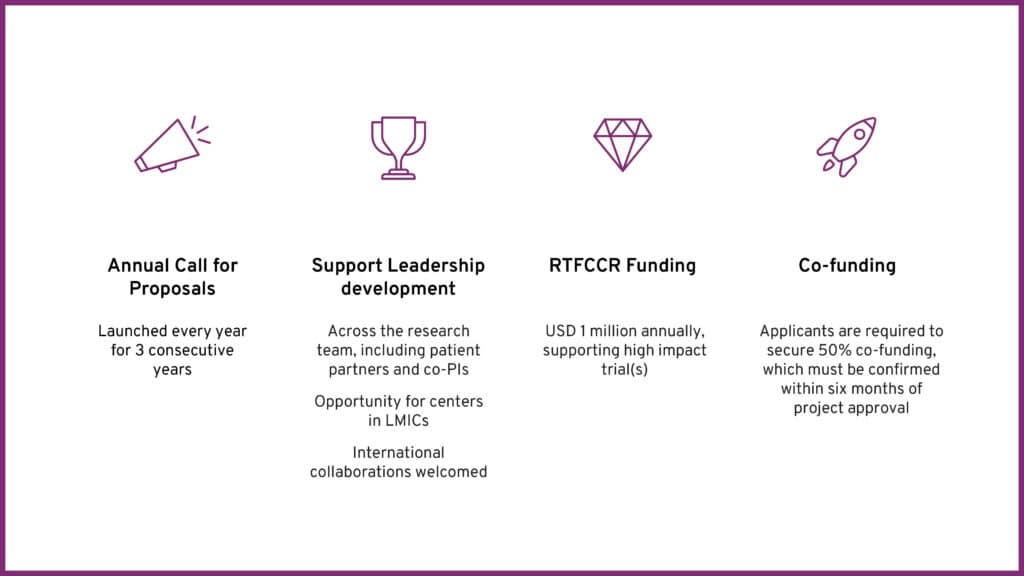
- Up to USD 500,000 per award (RTFCCR will cover 50% of the project total cost) for a maximum of 5 years.
- Applicants must secure 50% co-funding, confirmed within six months of approval.
Evaluation Criteria
Innovation
Novel approaches with high potential impact.
Scalability
Feasibility in diverse settings, especially LMICs.
Patient Engagement
Meaningful involvement in design and dissemination.
Scientific Merit
Robust methodology and clear milestones.
Application Process
- Letter of Intent (LOI): Submit via the online portal by March 09, 2026.
- Full Proposal: Invited applicants will receive detailed instructions.
- Review: Independent panel including scientific experts, patient partners, and biostatisticians.
Applicants can apply for RTFCCR funding opportunities through the online grant management system Smart Simple. The portal manages the entire grant process, including progress reports and awarded project payments.
Key Dates
- LOI Deadline: March 09, 2026
- Full Proposal Deadline: April 30, 2026
- Award Notification: August 2026
Contact
For guidelines and submission details, visit risingtide-foundation.org or email rtfccrteam@risingtide.ch
ACCESS Award
Examples of projects we already fund
ARCHERY
Radiotherapy is vital in cancer care, curing about 40% of cases and needed by half of all patients. Yet in many low- and middle-income countries, only 10–40% can access it. A major barrier is the time and expertise required for planning and delivery, leading to delays, patient stress, and poorer outcomes.
That’s where the ARCHERY study comes in. It’s testing a new AI tool called the Radiotherapy Planning Assistant (RPA) to help speed up and improve the planning process.
The RPA tool can:
- Automatically mark areas to treat and protect healthy parts of the body.
- Help design the radiation beams used in treatment.
If successful, this could:
- Cut planning time from hours to minutes
- Make treatment more consistent and safer
- Free up staff to focus on patient care
- Help make radiotherapy more affordable
ARCHERY is a large international study led by Ajay Aggarwal involving teams in India, Jordan, Malaysia, and South Africa. It focuses on prostate, cervical, and head and neck cancers, where radiotherapy is often the main treatment.
Patient Partner Involvement
- Patient partners have co-developed the study protocol, including patient information and consent forms.
- Patient partners have been part of the overarching study management group for the study, also evaluating reasons for participants declining recruitment to the study to inform future research.
Terbinafine for Biochemically Recurrent Prostate Cancer - A Phase II Drug-repurposing Study
Despite surgery or radiotherapy, many men with prostate cancer experience recurrence. When PSA levels rise and imaging does not yet show metastases (biochemical relapse), anti-hormonal therapy is typically initiated. While effective, these treatments often lead to significant side effects such as hot flushes, muscle weakness, sexual and cognitive impairment, and psychological distress—greatly reducing quality of life.
The study aims to test whether Terbinafine, a well-tolerated and affordable antifungal drug, can slow prostate cancer progression without hormone withdrawal. Because Terbinafine is widely available and inexpensive, it could be easily implemented in low- and middle-income countries (LMICs), where access to advanced cancer therapies is often limited.
Terbinafine has the potential to:
- Target an enzyme (SQLE) implicated in cancer growth.
- Delay the need for anti-hormonal therapy and its associated side effects.
If successful, this approach could:
- Improve patients’ quality of life
- Postpone hormone therapy for months or even years
- Provide a cost-effective option for LMICs
- Open new research avenues for advanced prostate cancer and other cancers
The study prospectively treats patients with rising PSA after local therapy using Terbinafine at two dose levels for up to one year, without additional hormone therapy. PSA will be monitored regularly, and imaging will be performed at the end of treatment to assess efficacy.
Patient Partner Involvement
- Patient partners have been permanently assisting core team meetings of the urogenital project group.
The Patient Advisory Board has provided guidance on issues such as study endpoints, interim analyses, and other related considerations.
Innovative Treatment for Cervical Pre-Cancer: Expanding Access in Low-Resource Settings
Cervical cancer is one of the most preventable yet deadly diseases among women in low- and middle-income countries (LMICs), primarily caused by persistent HPV infection. While screening technologies have advanced, treatment options for pre-cancerous lesions remain limited. In many low-resource settings, the standard treatment—gas-based cryotherapy—requires cryogenic gas tanks that are costly, difficult to transport, and pose safety risks.
That is where this clinical trial came in. The research team tested the LMIC-adapted CryoPen®, a cryotherapy device designed for these settings. It eliminates the need for cryogenic gas and can run on electricity or even a car battery. However, challenges remain:
- The device is heavy, limiting portability
- Requires two hours to cool before use
- Has a 15-minute waiting time between patients
- Needs ethanol as a consumable
To overcome these limitations, the team evaluated thermoablation—an alternative treatment that uses heat to destroy pre-cancerous lesions. Although thermoablation has been used since the 1970s, there are no clinical trials assessing its safety and efficacy. Despite this, several programs have adopted it because it is practical and straightforward.
The trial has successfully:
- Assessed thermoablation as a safe, non-invasive, and effective alternative to cryotherapy across a total of 1,131 women in El Salvador, Colombia, and China
- Tested a new handheld thermoablation prototype that operates on an external battery
- Generated evidence that can eventually inform global treatment guidelines
Thermoablation can provide a low-cost, portable solution for thousands of women in remote and underserved areas. This research focused on feasibility and impact in vulnerable communities, where traditional treatment methods are difficult to implement due to geographic, economic, and infrastructure constraints.