Advancing Cancer Research in Sub-Saharan Africa
This grants program, initially focused on Uganda and Kenya, has served as a pioneering initiative to advance clinical cancer research for rapid impact on patient outcomes in sub-Saharan Africa. It has also enhanced the capacities of applicants from these countries, empowering them to effectively compete as leading investigators for global research opportunities in the future. We are sharing the lessons learned in patient engagement from this pilot so they can serve as exemplary models for other African countries.
Approach
Concentrated efforts within a specific region rather than multiple countries and topics.
Ensured that chosen countries were committed and prepared to advance cancer initiatives.
Prioritized countries with stable infrastructure.
Leveraged technology for cancer control: diagnostics, telemedicine, image analysis, pattern recognition.
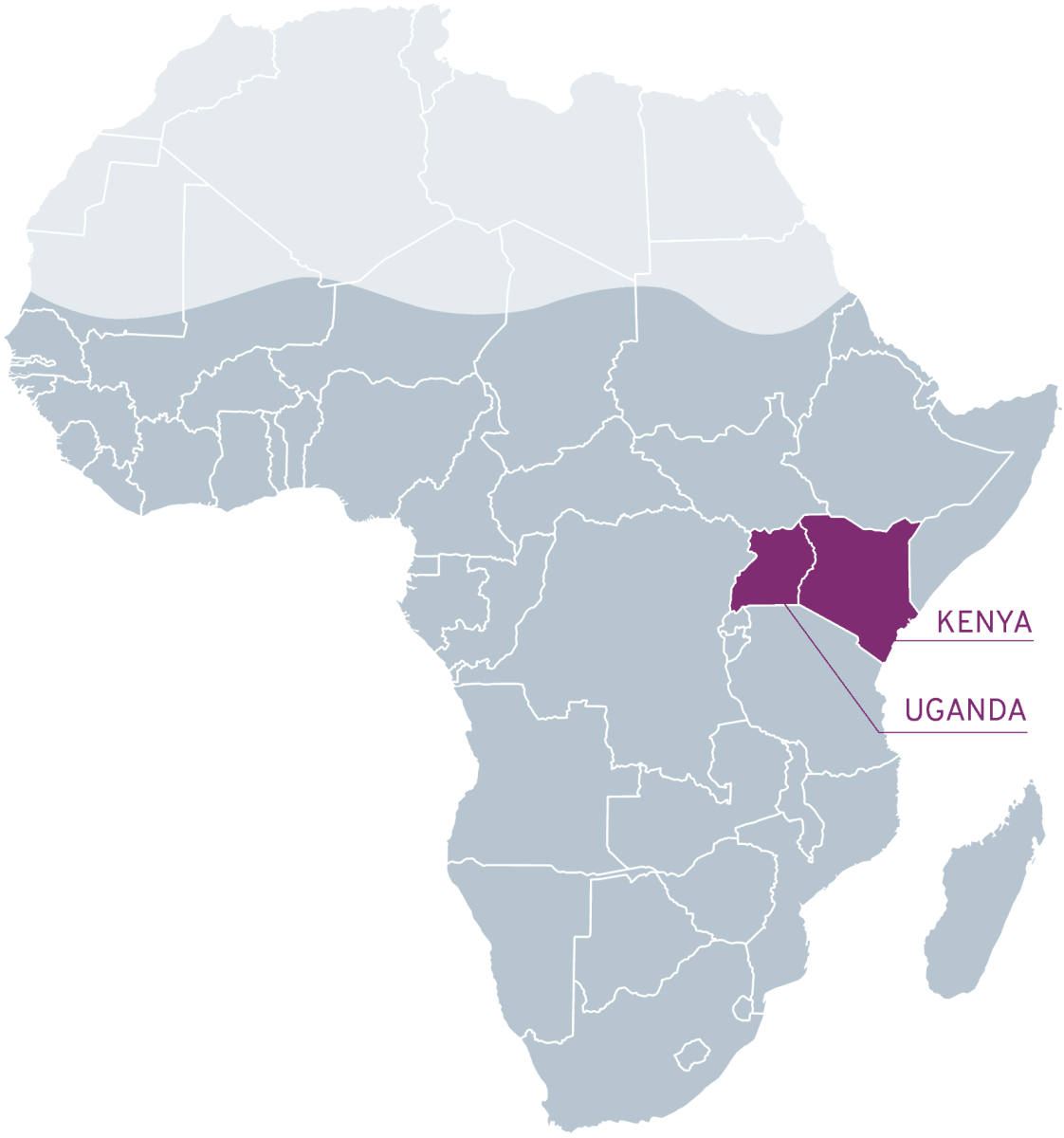
Area selection
21/25
Among the 25 countries with the highest cancer-related mortality rates globally, 21 are situated in sub-Saharan Africa
Uganda and Kenya, two prominent sub-Saharan African countries, have benefited immensely from substantial investments in supply chain management, clinical services, patient engagement, and medical research, thanks to the President’s Emergency Plan for AIDS Relief (PEPFAR). Leveraging this robust foundation, RTFCCR has chosen to launch its pilot program in these nations.
Supported Research Approaches and Permissible Technical Foci
This program supported cancer intervention in areas such as early detection and diagnosis, treatment, care delivery, and palliative care.
Emphasis on the use of generics, off-label, and/or other reasonably available drugs not yet approved within national guidelines for cancer, but for which safety data exists. This may include exploring low-dose Immuno-Oncology therapy (I-O) where the patient management infrastructure can adequately support.
Focus on patient and/or provider beliefs, social context, and/or practices that may be modified to improve patient outcomes. Examples include interventions to support treatment adherence, modifying provider practice to introduce screening, or addressing patient access barriers. Behavioral research is critical to prevention and detection efforts.
Policy and practice differ substantially in many African countries. Oncology National Treatment Guidelines have been recently adopted in some countries (Tanzania, 2020; Kenya, 2019); while others rely on brief guidance included in overall medical guidelines (Malawi, Uganda). There is a need for implementation research to move policy to practice across varied contexts and infrastructure.
Applications focused on breast, colorectal, esophageal, or hematological cancers were encouraged as opposed to those largely supported under global infectious disease investments (such as cervical or Kaposi Sarcoma).
RTFCCR was interested in research leading to better patient outcomes in the near-term future, including quality of life, with an emphasis on outcomes identified in partnership with patients. Implementation research and clinical trials, both biomedical and behavioral, were supported under this program. Proposals for basic and/or translational research were not invited.
Eligible Applicants
Applicants must have been based in Kenya or Uganda and affiliated with registered organizations (individual applications were not accepted) with prior experience implementing IRB or externally funded research.
They should also have demonstrated access to patient populations for advisory roles and future study participation.
Program Framework: Stages
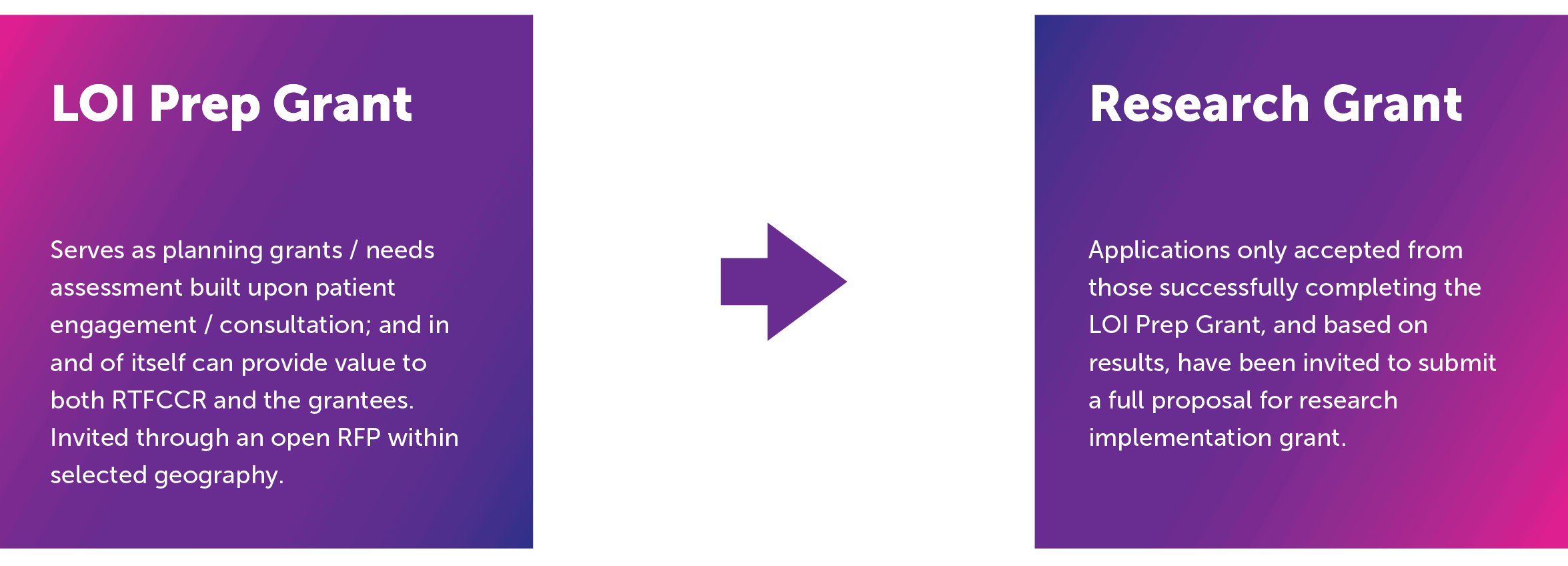
1. Pre-application grant
This phase aimed at conducting a meaningful assessment of needs and/or opportunities for cancer research (in prevention, detection, or treatment (including palliative care) that included patients not only as subjects but also as partners.
Assessments included focus groups, surveys, interviews, data analysis, etc. – but at a minimum, must have substantially and directly involved hearing patient voice. (Note –pre-application grants were not meant for research, but rather needs assessment, and usually did not require IRB approval; however, applicant institutions must have had experience with IRB-approved research.)
Below are the summaries of the six selected pre-application grants
2. Research grant
Implementation of clinical cancer research with patients as partners based on LOI Prep grant work/results.

